The UK has become the world’s first country to authorize a CRISPR gene-editing treatment for two inherited blood disorders, marking a significant milestone in medical science. The Medicines and Healthcare products Regulatory Agency (MHRA) has approved the therapy, named Casgevy (exagamglogene autotemcel), for patients aged 12 and over after rigorous safety and effectiveness assessments.
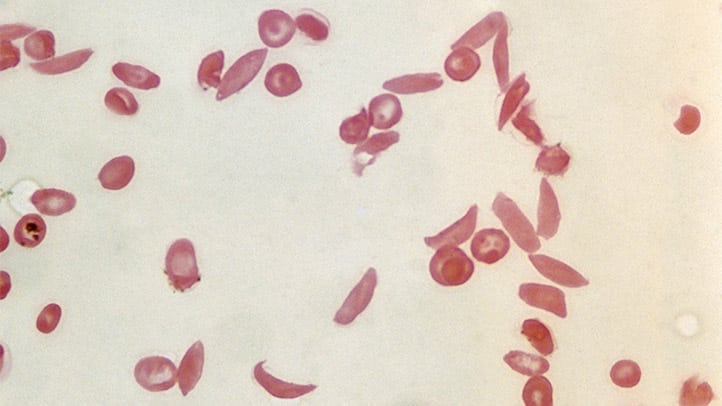
Developed by Vertex Pharmaceuticals and CRISPR Therapeutics, Casgevy targets sickle-cell disease and transfusion-dependent beta-thalassaemia. The treatment involves genetically editing a patient’s bone marrow stem cells to produce healthy hemoglobin, which is then infused back into the patient. This innovative approach could replace the only long-lasting treatment available until now: bone marrow transplants, which are highly arduous and carry significant side effects.
The decision by the UK’s regulator is based on promising clinical trial results, including significant reductions in pain and transfusion requirements for treated patients. However, the cost-effectiveness of this therapy is still under review to determine if it can be offered through the National Health Service.
While the treatment price remains undisclosed in the UK, it is part of a global initiative to make gene therapies more accessible. For instance, a recent gene therapy approved in Britain had a list price of £2.8 million ($3.5 million), but a confidential discount was negotiated to make it available to patients. In the U.S., the therapy’s cost is still being evaluated, with estimates suggesting a price up to around $2 million could be cost-effective.
This approval is a beacon of hope for millions worldwide, including about 100,000 in the U.S., who suffer from sickle cell disease. The condition is particularly prevalent in people with African, Middle Eastern, and Indian descent, often in regions historically affected by malaria. Carriers of the sickle cell trait are believed to have a protective advantage against severe malaria.
Casgevy’s approval represents not only a triumph in medical innovation but also a step towards equitable healthcare, potentially transforming the lives of those affected by these debilitating blood disorders.